How to determine the purity of oxygen?
- Copper wire
- Ammoniacal ammonium solution
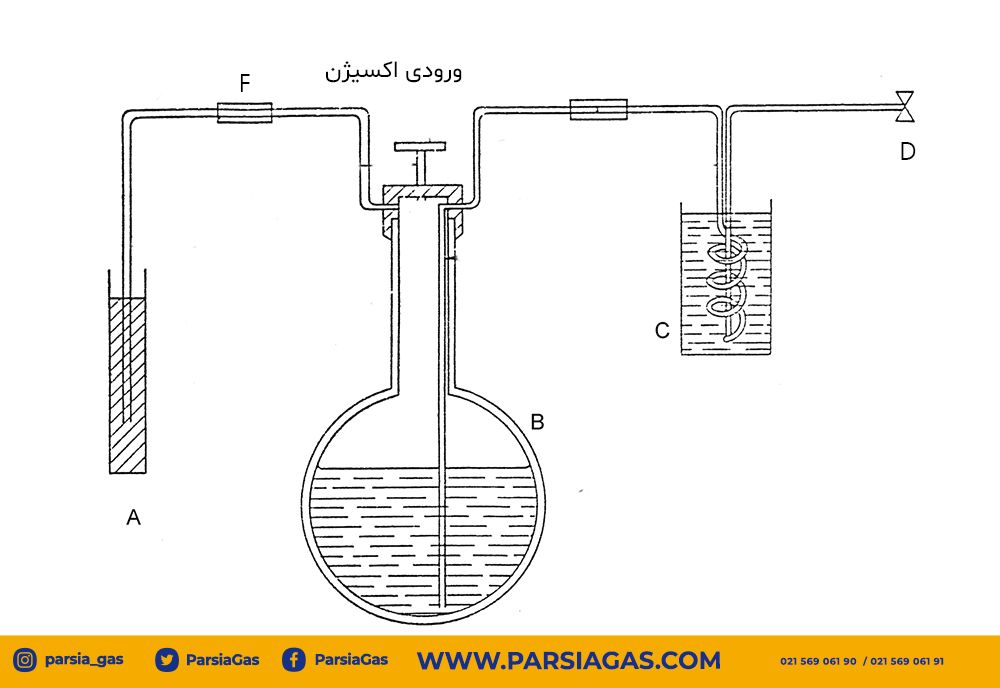
Before starting the work, adjust the amount of oxygen gas input to be tested and tank C, which contains copper wire, turn the tank upside down and close its lid tightly (tank C should be filled with spiral copper wire or any other shape.)
Then, tank C is filled with ammonia table and placed in the system, and container A, which is filled to about 3/4 of ammonia, is placed in a position where the buret (container B) is completely filled with ammonia solution and after This step connects the desired oxygen gas inlet hose to the system so that the oxygen gas enters container B and then opens the buret valve so that the oxygen from container B enters tank C and reacts with the copper wires in container C for ease of transfer. It is better to move the oxygen gas from tank B to tank C so that tank C is filled with oxygen and the valve of tank B is closed.
When measuring the purity, we introduce 100 cc of oxygen into the burette to react with copper and ammonia solution.
Ammonia copper elixir is removed from the operating environment.
The empty volume is actually impurities.
Burette, two-way valve, to determine the volume of oxygen gas entered and remove excess ammonia solution, we have a container containing copper spirals and a volume control tank.
Basis of work:
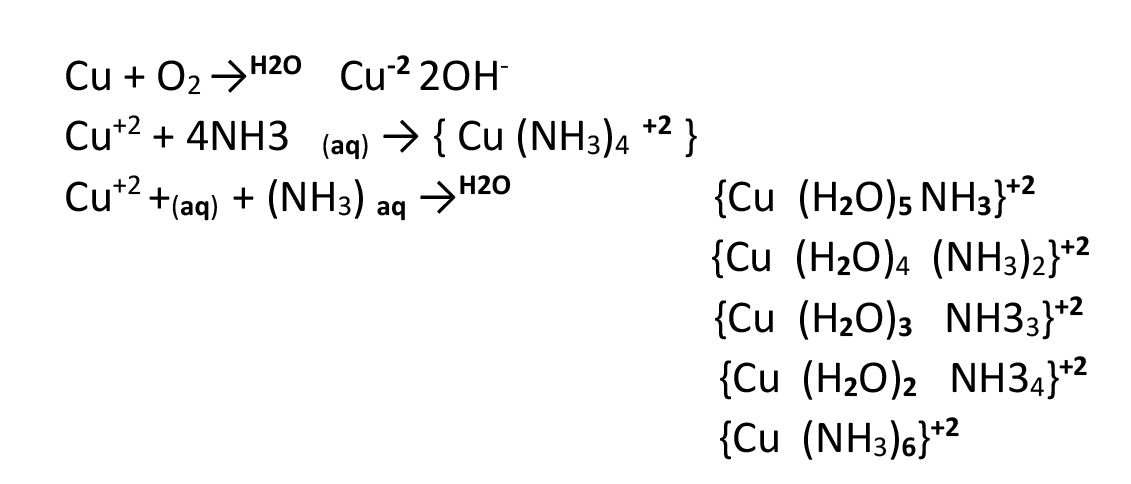
Oxygen reacts with copper metal in the presence of saturated ammonia solution and is absorbed by it.
Based on the initial volume of gas entered into the system and the final volume remaining after testing, the amount of oxygen gas impurities is determined based on volume percentage, based on which the purity of oxygen can be determined.
In some of the methods of this test, it is mentioned that 30 minutes should be given to this test so that oxygen can completely penetrate and act in tank C (the mechanism is that oxygen reacts with copper metal in the presence of saturated ammonia solution and is absorbed by it.) After 30 minutes, open the valve of container B again to connect tank C and container B again and obtain the impurity level of oxygen gas from B side.
- The system must be completely isolated.
- There should be no air penetration or leakage.
- The copper metal container should always be full.