Gas Chromatography
GC or gas chromatography is one of the common analytical tools in laboratories, especially in gas and oil laboratories, which is used to analyze materials, in a minimum time, to determine the purity and ratio of components of a compound that evaporate without decomposition during heating. Placed. In addition, the gas chromatography device is used in various industries such as agriculture, food, cosmetics, environment and pharmaceuticals.
The performance of the chromatography device is such that the analyte sample is usually injected into the system in a liquid state, and the components of an evaporated sample are separated by dividing between a gaseous mobile phase and a stationary phase.
The mobile phase is actually the carrier gas that contains the analyte sample. The carrier gas must be a chemically inert gas (including: helium, argon, carbon dioxide, nitrogen, and hydrogen) so as not to react with the analyte. It varies according to the type of detector. The stationary phase is an absorbent solid body or a thin layer of a non-volatile liquid that is placed on the inner wall of the column or as a coating on the surface of the glass or metal pellets.
Gas chromatography is divided into two categories: gas-liquid chromatography (GLC) and gas-solid chromatography (GSC). If the stationary phase is a non-volatile liquid, it is called gas-liquid chromatography, and if the stationary phase is an absorbent solid, it is called gas-solid chromatography. But both are known as gas chromatography.
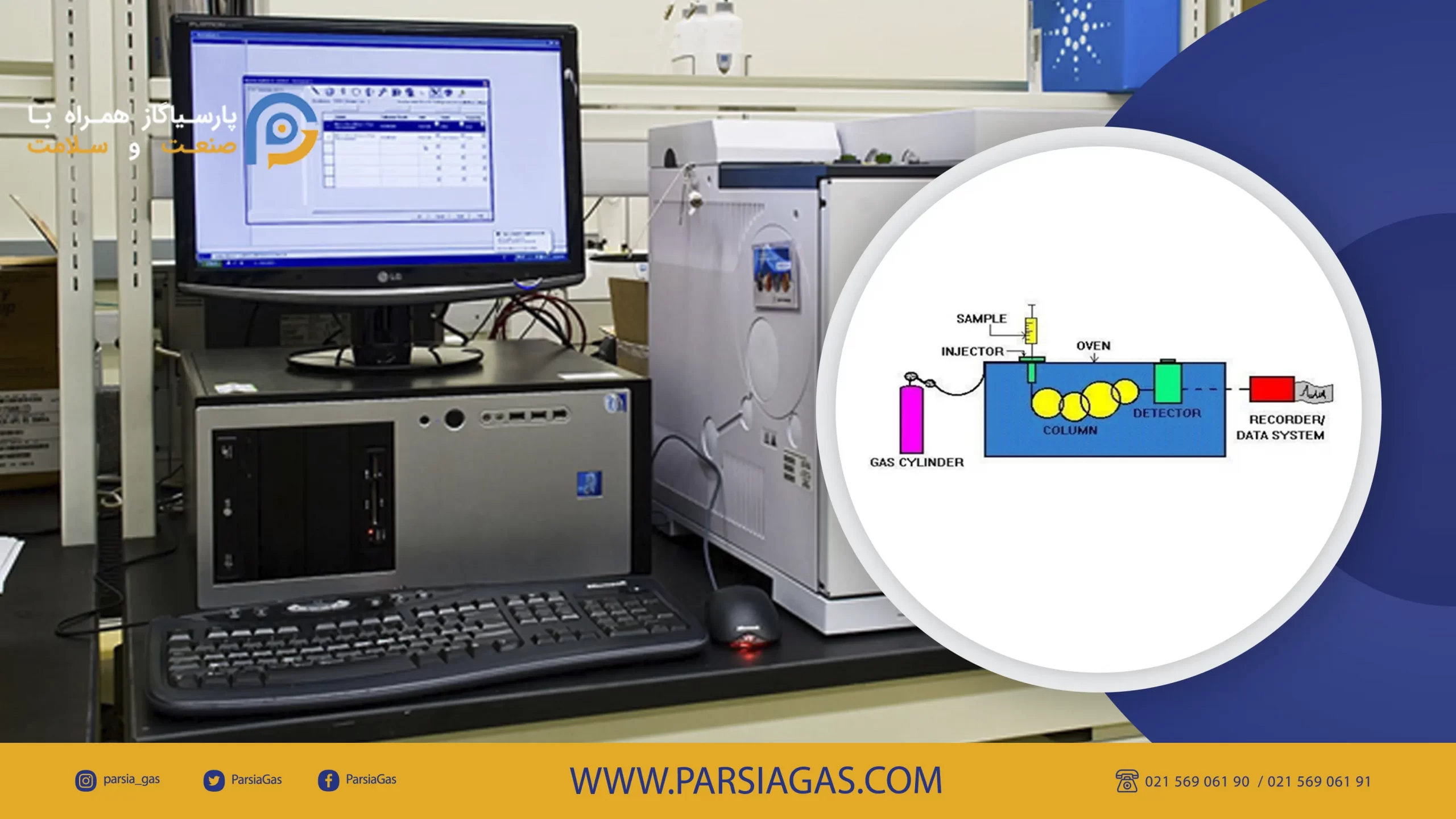
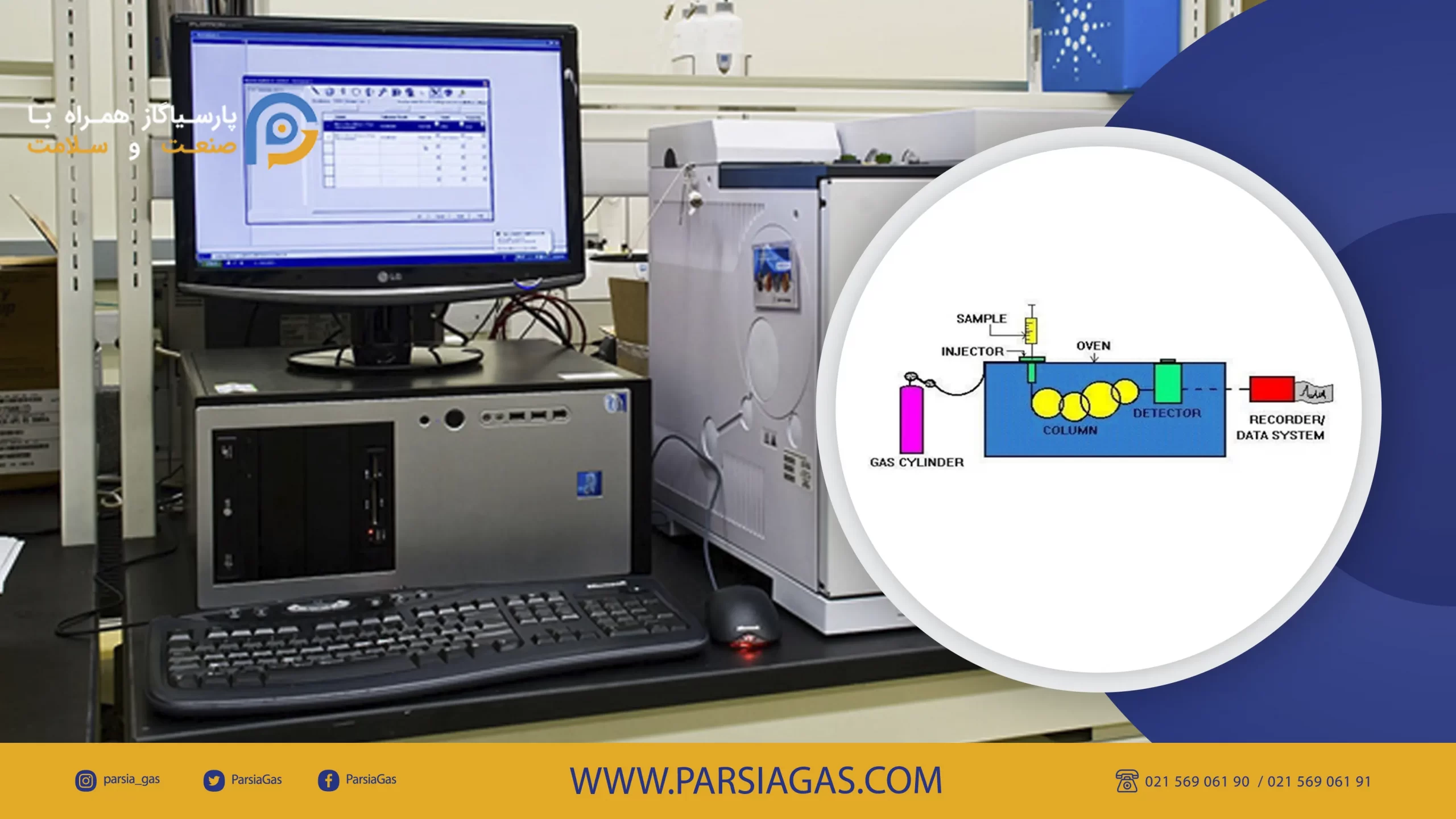
Schematic of gas chromatography device:
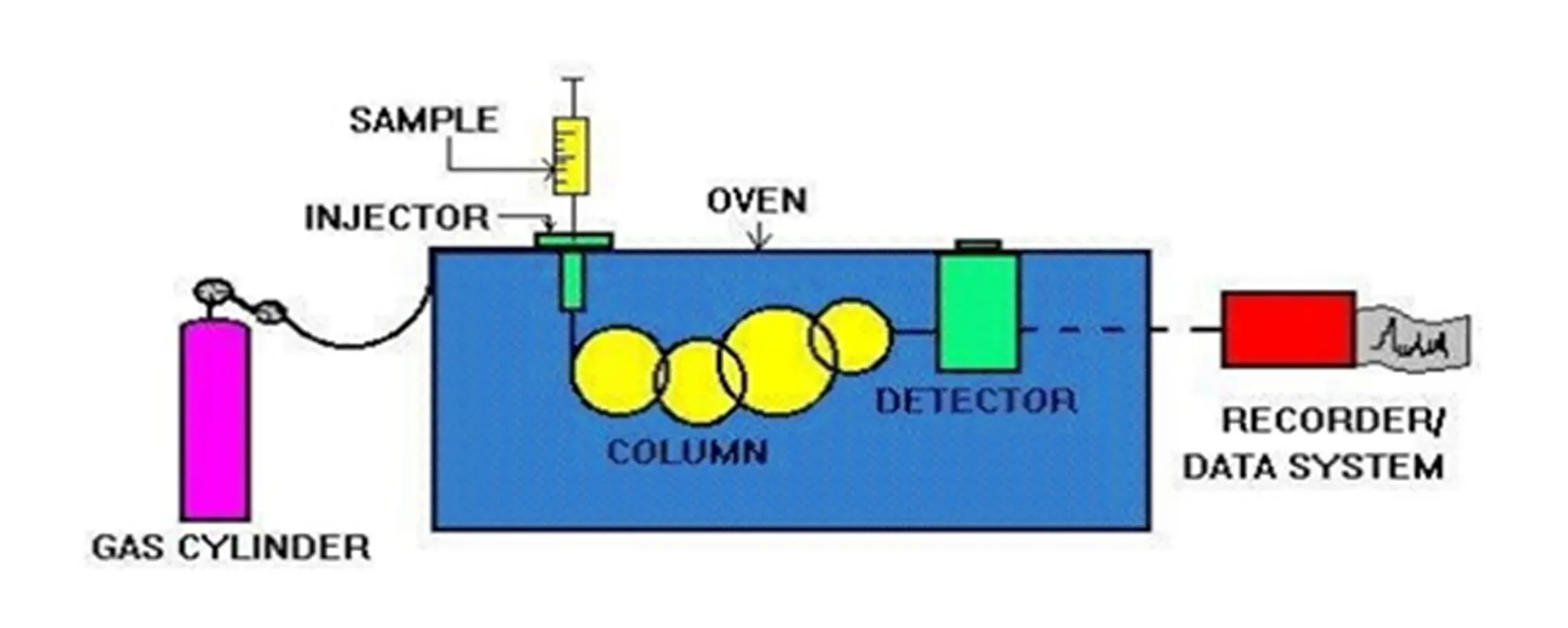
Different parts of gas chromatography device:
- Carrier Gas Cylinder
- Sample Injection Chamber
- Column
- Heater enclosures or oven
- Detector
- Displayer
Carrier gas:
The carrier gas must be of high purity and free of moisture and oxygen. The purity of the carrier gas is its most important feature, because the presence of impurities in the carrier gas causes disturbances and thus reduces the quantitative accuracy of the analysis.
The type of carrier gas has a great effect on the efficiency of the column and the analysis time. Helium gas is known as one of the most common carrier gases due to its good performance.
But the hydrogen carrier gas reduces the analysis time due to its high flow rate and low molecular weight, and it is also difficult to store hydrogen gas due to its explosive properties. However, because nitrogen gas is heavier than hydrogen gas in optimal volumes, they have a high performance, but it increases the analysis time.
Place of sample injection:
The carrier gas enters the chamber and after mixing with the vaporized sample, part of the composition enters the column and part leaves through the outlet (for separate compositions). In order to achieve a high column performance, the sample should be introduced into the column with the right amount into a mass of vapor at an optimal speed. Injection temperature is another important thing in injection systems that should be taken into account.
column:
The column is the most important part of chromatography devices, whose main task is to separate the components of the composition. There are two types of columns in a gas chromatography device: packed columns and capillary columns, which are filled columns made of glass, metal (stainless steel, copper, aluminum) or Teflon tubes. The column is filled with small particles that are completely inert and are covered by the liquid phase and form the stationary phase. Capillary columns, which have two types of wall-coated open tube columns (WCOT) and coated open tube columns (SCOT).
These columns are in the form of open tubes and most of the columns are made of glass, but stainless steel, copper, aluminum and plastic are also used. that the stationary phase is placed as a thin film layer on the inner wall of the column. Both of these columns are more efficient than filled columns.
Heating chamber (oven):
The oven is responsible for adjusting the temperature of the column during the execution of the temperature program. The temperature of the oven is set in two modes: Isothermal and Programmable. The optimal column temperature is slightly higher than the average boiling point of the sample.
In the isothermal method, the sample has one component or the components of the sample have a boiling point close to each other, in this case we give the oven a constant temperature. In the programmable method, the sample has multiple components, which have different boiling points, in this case, we must give it a temperature program.
Detector:
- mass detector (MSD)
- Flame Light (FPD)
- Photoionization (PID)
- Electrolytic Conduction (ELCD)
- Atomic emission detector (AED)